For Healthcare Professionals
Access these tools to help optimize the Rasuvo experience

Help your patients save
The Co-pay Assistance Program can provide financial support of up to $125 per month for 14 months.*
- 85% of patients who used the co-pay card had a $0 co-pay1,†
- Preferred on many formularies
- 84% of commercial lives covered, 46% preferred brand2
- Available on insurance plans serving over 156 million patients2
Rasuvo has the most comprehensive coverage of any scMTX Auto-Injector2,‡
- CVS Caremark®
- Express Scripts®
- Humana®
- Cigna®
- UnitedHealth Group®
- Highmark Health®
- HCSC®
- TRICARE®
- Anthem®
- OptumRx®
- Preferred
- Covered
*For eligible patients.
†Data from April 2016 to April 2017.
‡Average nationwide coverage of methotrexate auto-injectors. Individual coverage may vary.
All brand or product names are registered trademarks of their respective owners.
*For eligible patients.

Practice Resources
Utilize CORE Connections—a program that supports Rasuvo® (methotrexate) injection patients with educational materials, financial assistance, insurance forms, and more.

For reimbursement services and patient eligibility determinations, call the Rasuvo Benefits Support Line at 1-855-336-3322, from 9:00 AM to 6:00 PM EST Monday-Friday (excluding holidays).
Video practice resources
Show your patients a video demonstration.
See Auto-Injector: 3 Easy Steps Video
Auto-injector ease of use
Compared to the syringe and vial method of MTX administration, Rasuvo makes it easier to deliver MTX subcutaneously. The prefilled auto-injector eliminates the need for preparing doses by hand.3-5
3-Step Delivery of Rasuvo

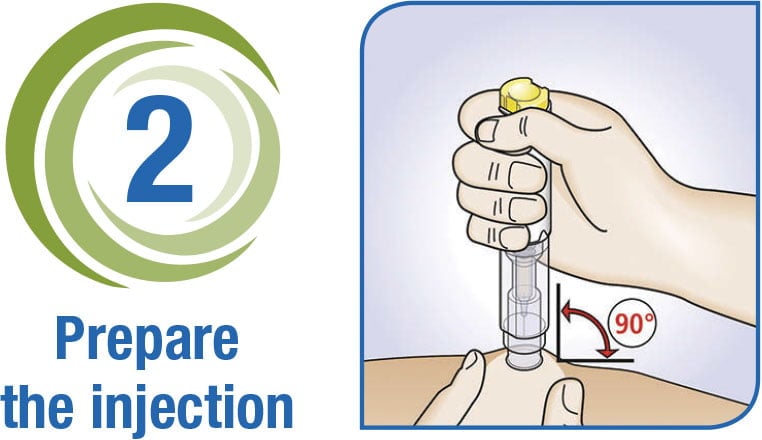
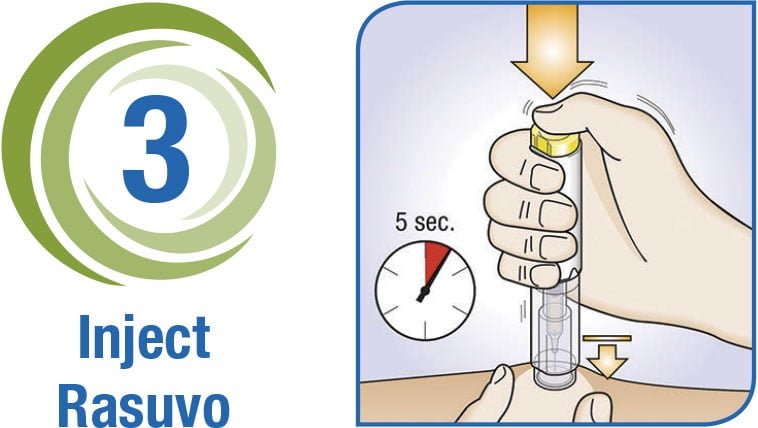
Download the Rasuvo Administration Guide for complete instructions on how to use the Rasuvo auto-injector pen.


References: 1. Data on file. Medexus Pharma, Inc. 2. Rheumatoid arthritis database. Yardley, PA: Managed Markets Insight & Technology, LLC (MMIT): August 2019. 3. Demary W, Schwenke H, Rockwitz K, et al. Subcutaneously administered methotrexate for rheumatoid arthritis, by prefilled syringes versus prefilled pens: patient preference and comparison of the self-injection experience. Patient Prefer Adherence. 2014;8:1061-1071. 4. Pachon JA, Kivitz AJ, Heuer KU, Pichlmeier U. Assessing usability, label comprehension, pen robustness and pharmacokinetics of a self-administered prefilled autoinjector pen of methotrexate in patients with rheumatoid arthritis. SAGE Open Med. 2014;2:1-12. 5. Rasuvo [prescribing information]. Chicago, IL: Medac Pharma, Inc.; 2018.
INDICATIONS:
Rasuvo is indicated for the:
- Management of adults with severe, active rheumatoid arthritis (RA) (ACR criteria) or children with active polyarticular juvenile idiopathic arthritis (pJIA), who are intolerant of or had an inadequate response to first-line therapy, including full dose non-steroidal anti-inflammatory agents (NSAIDs).
- Symptomatic control of severe, recalcitrant, disabling psoriasis in adults who are not adequately responsive to other forms of therapy, but only when the diagnosis has been established, as by biopsy and/or after dermatologic consultation. It is important to ensure that a psoriasis “flare” is not due to an undiagnosed concomitant disease affecting immune response.
Limitations of Use
Rasuvo is not indicated for treatment of neoplastic diseases.
This product includes the following Boxed Warning:
WARNING: SEVERE TOXIC REACTIONS, INCLUDING EMBRYO-FETAL TOXICITY AND DEATH
Rasuvo should be used only by physicians whose knowledge and experience include the use of antimetabolite therapy. Because of the possibility of serious toxic reactions (which can be fatal), Rasuvo should be used only in patients with psoriasis or rheumatoid arthritis with severe, recalcitrant, disabling disease which is not adequately responsive to other forms of therapy. Deaths have been reported with the use of methotrexate in the treatment of malignancy, psoriasis, and rheumatoid arthritis. Patients should be closely monitored for bone marrow, liver, lung, skin, and kidney toxicities. Patients should be informed by their physician of the risks involved and be under a physician's care throughout therapy.
- Methotrexate has been reported to cause fetal death and/or congenital anomalies. Therefore, Rasuvo is not recommended for females of childbearing potential unless there is clear medical evidence that the benefits can be expected to outweigh the considered risks. Rasuvo is contraindicated in pregnant women.
- Methotrexate elimination is reduced in patients with impaired renal functions, ascites, or pleural effusions. Such patients require especially careful monitoring for toxicity, and require dose reduction or, in some cases, discontinuation of Rasuvo administration.
- Unexpectedly severe (sometimes fatal) bone marrow suppression, aplastic anemia, and gastrointestinal toxicity have been reported with concomitant administration of methotrexate (usually in high dosage) along with some nonsteroidal anti-inflammatory drugs (NSAIDs).
- Methotrexate causes hepatotoxicity, fibrosis and cirrhosis, but generally only after prolonged use. Acutely, liver enzyme elevations are frequently seen. These are usually transient and asymptomatic, and also do not appear predictive of subsequent hepatic disease. Liver biopsy after sustained use often shows histologic changes, and fibrosis and cirrhosis have been reported; these latter lesions may not be preceded by symptoms or abnormal liver function tests in the psoriasis population. For this reason, periodic liver biopsies are usually recommended for psoriatic patients who are under long-term treatment. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis in the rheumatoid arthritis population.
- Methotrexate-induced lung disease, including acute or chronic interstitial pneumonitis, is a potentially dangerous lesion, which may occur acutely at any time during therapy and has been reported at low doses. It is not always fully reversible and fatalities have been reported. Pulmonary symptoms (especially a dry, nonproductive cough) may require interruption of treatment and careful investigation.
- Diarrhea and ulcerative stomatitis require interruption of therapy: otherwise, hemorrhagic enteritis and death from intestinal perforation may occur.
- Malignant lymphomas, which may regress following withdrawal of methotrexate, may occur in patients receiving low-dose methotrexate and, thus, may not require cytotoxic treatment. Discontinue Rasuvo first and, if the lymphoma does not regress, appropriate treatment should be instituted.
- Like other cytotoxic drugs, methotrexate may induce “tumor lysis syndrome” in patients with rapidly growing tumors.
- Severe, occasionally fatal, skin reactions have been reported following single or multiple doses of methotrexate. Reactions have occurred within days of oral, intramuscular, intravenous, or intrathecal methotrexate administration. Recovery has been reported with discontinuation of therapy.
- Potentially fatal opportunistic infections, especially Pneumocystis jiroveci pneumonia, may occur with methotrexate therapy.
- Methotrexate given concomitantly with radiotherapy may increase the risk of soft tissue necrosis and osteonecrosis.
CONTRAINDICATIONS
Rasuvo is contraindicated in the following:
- Pregnancy: Rasuvo can cause fetal death or teratogenic effects when administered to a pregnant woman. Rasuvo is contraindicated in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Nursing Mothers: Because of the potential for serious adverse reactions from methotrexate in breast fed infants, Rasuvo is contraindicated in nursing mothers.
- Alcoholism or Liver Disease: Patients with alcoholism, alcoholic liver disease or other chronic liver disease.
- Immunodeficiency Syndromes: Patients who have overt or laboratory evidence of immunodeficiency syndromes.
- Preexisting Blood Dyscrasias: Patients who have preexisting blood dyscrasias, such as bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia.
- Hypersensitivity: Patients with a known hypersensitivity to methotrexate. Severe hypersensitivity reactions have been observed with methotrexate use.
WARNINGS AND PRECAUTIONS
- Organ System Toxicity: Rasuvo has the potential for serious toxicity. Rasuvo should be used only by physicians whose knowledge and experience include antimetabolite therapy. Because of the possibility of severe toxic reactions (which can be fatal), Rasuvo should be used only in patients with psoriasis or rheumatoid arthritis with severe, recalcitrant, disabling disease which is not adequately responsive to other forms of therapy.
- Embryo-Fetal Toxicity: Methotrexate has been reported to cause fetal death and/or congenital anomalies. Rasuvo is not recommended for females of childbearing potential unless there is clear medical evidence that the benefits can be expected to outweigh the considered risks. Rasuvo is contraindicated in pregnant women with psoriasis or rheumatoid arthritis. Exclude pregnancy before treatment. Females should be counseled on the serious risks to the fetus should they become pregnant while undergoing treatment. Avoid pregnancy if either partner is receiving Rasuvo. Advise males to avoid pregnancy for a minimum of three months after therapy and females to avoid pregnancy for at least one ovulatory cycle after therapy.
- Effects on Reproduction: Methotrexate has been reported to cause impairment of fertility, oligospermia and menstrual dysfunction in humans, during and for a short period after cessation of therapy.
- Laboratory Tests: Patients undergoing Rasuvo therapy should be closely monitored so that toxic effects are detected promptly. Baseline assessment should include a complete blood count with differential and platelet counts, hepatic enzymes, renal function tests and a chest X-ray.
- Risks from Improper Dosing: The physician and/or pharmacist should emphasize to the patient that Rasuvo is administered once weekly and mistaken daily use has led to fatal toxicity.
- Patients with Impaired Renal Function, Ascites, or Pleural Effusions: Elimination is reduced. Such patients require especially careful monitoring for toxicity and require dose reduction or, in some cases, discontinuation of Rasuvo administration.
- Dizziness and Fatigue: May impair ability to drive or operate machinery.
- Malignant Lymphomas: Non-Hodgkin’s lymphoma and other tumors have been reported in patients receiving low-dose oral methotrexate. However, there have been instances of malignant lymphoma arising during treatment with low-dose methotrexate, which have regressed completely following withdrawal of methotrexate, without requiring active anti-lymphoma treatment. Discontinue Rasuvo first and, if the lymphoma does not regress, appropriate treatment should be instituted.
- Tumor Lysis Syndrome: Like other cytotoxic drugs, methotrexate may induce “tumor lysis syndrome” in patients with rapidly growing tumors.
- Concomitant Radiation Therapy: Methotrexate given concomitantly with radiotherapy may increase the risk of soft tissue necrosis and osteonecrosis.
ADVERSE REACTIONS
Common adverse reactions are: nausea, abdominal pain, dyspepsia, stomatitis/mouth sores, rash, nasopharyngitis, diarrhea, liver function test abnormalities, vomiting, headache, bronchitis, thrombocytopenia, alopecia, leukopenia, pancytopenia, dizziness, photosensitivity, and “burning of skin lesions”. The most frequently reported adverse reactions include ulcerative stomatitis, leukopenia, nausea, and abdominal distress. Other frequently reported adverse reactions are malaise, undue fatigue, chills and fever, dizziness and decreased resistance to infection.
DRUG INTERACTIONS
- Aspirin, NSAIDs, and Steroids: Concomitant use may elevate and prolong serum methotrexate levels, resulting in deaths from severe hematologic and gastrointestinal toxicity.
- Proton Pump Inhibitors (PPIs): Concomitant use of some PPIs may elevate and prolong serum levels of methotrexate and cause increased toxicity.
- Oral Antibiotics: Certain oral antibiotics may decrease intestinal absorption of methotrexate or interfere with enterohepatic circulation by inhibiting bowel flora and suppressing metabolism of the drug by bacteria. Use of Rasuvo with penicillins should be carefully monitored.
- Hepatotoxins: Patients receiving concomitant therapy with Rasuvo and other potential hepatotoxins should be closely monitored for possible increased risk of hepatotoxicity.
- Theophylline: Methotrexate may decrease the clearance of theophylline, therefore theophylline levels should be monitored.
- Folic Acid and Antifolates: Vitamin preparations containing folic acid or its derivatives may decrease responses to systemically administered methotrexate. Folate deficiency states may increase methotrexate toxicity.
- Mercaptopurine: Methotrexate increases the plasma levels of mercaptopurine, therefore dose adjustment may be required.
- Nitrous Oxide: The use of nitrous oxide anesthesia potentiates the effect of methotrexate on folate dependent metabolic pathways, resulting in the potential for increased toxicity. Avoid the simultaneous use of nitrous oxide and methotrexate.
- Other Drugs: Toxicity may be increased because of displacement of certain drugs. Use of Rasuvo with probenecid should be carefully monitored.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Methotrexate has been reported to cause embryotoxicity, fetal death, congenital anomalies, and abortion in humans and is contraindicated in pregnant women.
- Nursing Mothers: Because of the potential for serious adverse reactions from methotrexate in breast fed infants, Rasuvo is contraindicated in nursing mothers.
- Females and Males of Reproductive Potential: Contraception should be used by both females and males while taking Rasuvo. Pregnancy should be avoided if either partner is receiving Rasuvo:
- for a minimum of 3 months after treatment with Rasuvo for males.
- during and for at least 1 menstrual cycle after treatment with Rasuvo for females.
- Pediatric Use: Safety and efficacy of Rasuvo have not been established in pediatric patients with psoriasis or neoplastic disease.
- Geriatric Use: Use caution in dose selection. Elderly patients should be closely monitored for early signs of hepatic, bone marrow and renal toxicity.
- Renal Impairment: Elimination is reduced. Patients require careful monitoring for toxicity and require dose reduction or discontinuation of Rasuvo.
- Hepatic Impairment: Contraindicated in patients with alcoholic liver disease or other chronic liver disease.
DOSAGE AND ADMINISTRATION
Rasuvo is for once weekly subcutaneous use only.
Administer Rasuvo in the abdomen or thigh.
March 2020
